Updated 08/29/23
Now that projects are on their way back, I’ll like to introduce
The Fascia System into the mix as it’s a connective tissue that envelops, separates, and stabilizes muscles and other internal organs.
The influence of mechanical forces on Fascia?
Connective tissue / fascia is an incredibly adaptable and plastic tissue. It is transformed, remodeled and strengthened or weakened according to the mechanical stimulation (load) to which it is exposed. If we don’t move, the tissue will diminish.
Different type of mechanical stress provides different stimuli to the tissue cells.
Depending on the type of load, the cells are stimulated to produce different constituents in the connective tissue, such as different types and amounts of collagen and ground substance (different glucosaminoglycans and proteoglycans, including hyaluronan).
- This means that in a tendon that is loaded (stretched and extended), the cells are stimulated to form more fibers of collagen type I, which is the most common type in tendons, ligaments and muscle fascia. It is also the strongest type of collagen, which forms strong fibers, which lie parallel in the direction of the mechanical load. This stretch also stimulates an enzyme, lysyl oxidase, which helps to create cross-links between the collagen fibers, to make the tissue stronger, a tendon for example. The more tensile stimulation, the more collagen type I and the more crosslinks.
- The collagen in a joint that is loaded, is compressed, and then the pressure lightens. This dynamic compression of the cells stimulates them to form more collagen type II. Collagen type II is the type of collagen that is mainly found in cartilage.
- In loose fascia, the superficial fascia and between layers of dense fascia in the myofascial, where a lot of sliding is needed between two surfaces, shear forces take place. This type of mechanical force stimulates production of collagen type I and III in approximately equal amounts and there is also a need for more ground substance, including hyaluronic acid, which binds water and provides the sliding function. Hyaluronic acid is produced by special cells, fasciacytes, which are located just in the boundary area between two sliding layers.
What happens if a tendon’s not being used?
When a tendon is not used, if the load ceases, the tissue will decrease in size and it will be transformed and have a more scar-like structure. The number of cells will increase and after some weeks there are five times as many cells in the tissue. The collagen fibers decrease in size and after six weeks have almost halved their size. They will also cease to lie parallel in the direction of the mechanical load because there is no force direction as the tendon is not loaded. Instead, the fibers lie more disorderly, just like a scar. When the tendon is to be used again, it must be carefully adjusted to the load again, not to split.
A recent research study (Myrick et al, 2019) has investigated how ligaments adapt to intense training and strain in female football players. With the help of MRI, the volume of the anterior cruciate ligament has been measured after the end of the competition season compared with the same measurement before the start of the season. The volume of the cruciate ligament increased during the competition season and the difference was greatest in the “kick leg”. During the more intense load period, the players probably received continuous micro-damage, which led to inflammation, proliferation of cells and increased collagen production. The tissue tries to adapt to the increased mechanical force.
Source:
https://fasciaguide.com/research/hur-formas-fascia-av-belastning/
- Superficial Fascia (also known as subcutaneous tissue or hypodermis):
- Location: Directly beneath the skin.
- Composition: This layer consists of a mix of fibrous connective tissue, fat cells, blood vessels, and nerves.
- Function: It provides insulation and padding, acts as an energy storage medium due to the presence of fat cells, allows for mobility over deeper structures, and provides a pathway for blood vessels and nerves to travel to the skin.
- Deep Fascia:
- Location: Deeper than the superficial fascia, the deep fascia surrounds and infuses with muscles, bones, nerves, and blood vessels.
- Composition: Dense fibrous connective tissue, which is highly organized and mostly devoid of fat. It can vary in thickness, depending on its location and function.
- Function: This fascial layer stabilizes and separates individual muscles and muscle groups, allowing them to function independently. It also transmits force from muscles while limiting the spread of infectious or harmful agents to adjacent structures. Some parts of the deep fascia, like the iliotibial band, can be very thick and can themselves function as ligaments.
- Visceral (or Deep Visceral) Fascia:
- Location: Surrounds the internal organs, including those within the chest (like the heart and lungs) and abdomen (like the liver, spleen, and intestines).
- Composition: Delicate, thin connective tissue layers which are often accompanied by a thin layer of smooth muscle.
- Function: Visceral fascia helps to suspend and position the internal organs within their respective cavities, and provides a slippery surface so organs can move against each other (e.g., during digestion or breathing). It also contains the blood vessels, lymphatics, and nerves that supply the organs.
The organization and integrity of the fascial system are imperative for proper biomechanical function, and its health is maintained through a complex interplay of various cellular activities, tissue properties, and extracellular matrix (ECM) compositions.
1. Cellular Activity:
- Fibroblasts: These are the primary cells in the fascial system. They produce the essential components of the extracellular matrix like collagen, elastin, and proteoglycans. Their activity is influenced by mechanical signals, hormonal factors, and other local cell-cell interactions. When there’s a need for repair or adaptation (like in response to physical training), fibroblasts produce more ECM components to reinforce the tissue.
- Myofibroblasts: These are specialized fibroblasts that have properties of both fibroblasts and smooth muscle cells. They play a crucial role in wound healing by contracting and bringing the edges of a wound together.
2. Extracellular Matrix (ECM):
- Collagen: The primary structural protein in fascia, collagen provides tensile strength. The organization of collagen fibers depends on the specific demands of that tissue. For instance, in tendons, collagen fibers align in the direction of force application for maximum strength. In other fascial tissues, collagen might be more randomly organized to offer multi-directional support.
- Elastin: Provides elasticity, allowing the tissue to return to its resting shape after being stretched.
- Proteoglycans and Glycosaminoglycans (GAGs): These molecules fill spaces between the fibrous proteins in the ECM, providing hydration and resistance to compression.
3. Mechanical Loading:
- Mechanotransduction: This is the process by which cells sense and respond to mechanical forces. In the context of fascia, when mechanical loads are applied, cells within the tissue (like fibroblasts) sense these forces and respond by adjusting their metabolic activity. This can result in the remodeling and reinforcement of fascial tissues. Regular and appropriate mechanical loading, as seen in exercise or manual therapy, can optimize fascial organization and health.
- Piezoelectric Effect: Fascia exhibits a piezoelectric effect, which means it generates an electric charge in response to mechanical stress. This can influence cellular activities and has been proposed as a mechanism that could guide tissue repair and remodeling.
4. Fluid Dynamics:
- Movement and mechanical loading also influence fluid dynamics within fascial tissues. Proper fluid movement ensures nutrient delivery, waste removal, and can even influence the sliding and gliding mechanisms of fascial layers against one another.
A “highly trained” fascial system refers to one that has adapted to regular and specific demands, leading to improved function and resilience.
-
Increased Tensile Strength: Just as muscles grow stronger with training, the fascia also adapts to become more resistant to tears and strains. Regular loading and stretching can promote the alignment of collagen fibers in ways that improve the tensile strength of the tissue, making it more resistant to injuries.
-
Improved Elastic Recoil: A well-trained fascial system can store and release energy efficiently, much like a spring. This property can enhance athletic performance, especially in activities requiring explosive movements like jumping or sprinting.
-
Enhanced Proprioception: Fascia is rich in nerve endings, including proprioceptors that provide feedback about body position. A trained fascial system can improve proprioception, leading to better body awareness, balance, and coordination.
-
Reduced Risk of Injury: Regular training and conditioning of the fascial tissues can improve their flexibility and resilience. This adaptability reduces the risk of fascial injuries, which can often be debilitating and slow to heal.
-
Improved Sliding and Gliding Mechanisms: The layers of fascia need to slide and glide over each other smoothly for optimal movement. Training can maintain the hydration and lubrication of these layers, preventing adhesions and ensuring smooth, unrestricted movement.
-
Optimal Fluid Dynamics: Regular movement and loading ensure that fluids within the fascial tissues are consistently circulated. This not only delivers nutrients but also helps in the removal of waste products.
-
Adaptive Thickening or Thinning: In areas of high demand or specific loading, fascia can thicken for added support (e.g., plantar fascia in runners). Conversely, in areas where flexibility and adaptability are required, it might remain thin and supple.
-
Enhanced Healing and Regeneration: A trained fascial system might respond more efficiently to minor damages, thanks to improved circulation and the proactive activity of fibroblasts.
-
Positive Neurological Responses: Fascial manipulation, as seen in certain therapeutic modalities, has been proposed to lead to neurological relaxation responses, pain modulation, and muscle tone regulation.
-
Efficient Movement Patterns: A well-adapted fascial system contributes to the optimization of movement patterns, reducing unnecessary strain on joints and muscles and conserving energy.
Importance of Collagen and Fascia:
Collagen is the second most abundant substance in the Body (Water being number one)
It provides structural support for organs and soft tissue and levels of production decrease as you age.
Joints and muscles are bound together by connective tissues (tendons, ligaments, cartilage, intramuscular and Fascia)
When we apply force to anything, it’s through these connective tissues that that force is transferred through up to 80% of muscular force transfer to the connective tissue. Basically, they act as shock absorbers to the joints. The more you have the better, most are made of Type 1 collagen.
If you lift weights with deliberately slow movements you can improve the strength of individual collagen fibers allowing the body to rebuild much stronger and more resilient connective tissue structures.
good examples could be Eccentric or Isometric training.
Isometric training is when force is applied to a muscle/s but the muscle length is not changed.
Eccentric training is where the muscle lengthens under load or tension.
Another critical benefit of isometric and eccentric exercise is its ability to align the collagen fibers correctly within the connective tissue. Healthy collagen tissue creates a cross-weaving type of pattern leading to a stronger structure for movement, whereas abnormal weaving is weak and prone to re-injury.
Scar tissue interferes with the process after the injury to heal correctly. The exact reasons are unknown but some correlation has been linked to continually moving poorly, poor nutrition, and lack of quality rest. Until the scar tissue is replaced with healthy cells full recovery is unlikely and constant reinjuring is likely.
Old research:
Solution(s)
- Using a combination of recombinant Periostin (rPOSTN), a protein critical for tissue repair, and Aligned Collagen Fiber (ACF) scaffolds. It was found that rPOSTN delivery in ACF scaffolds substantially improved tendon structure, with enhanced collagen alignment and superior mechanical properties.
This research paper focuses on the utilization of recombinant Periostin (rPOSTN) loaded onto an aligned collagen fiber (ACF) scaffold for the effective regeneration of Achilles tendons. The key findings and their implications are as follows:
-
rPOSTN Enhances Tendon Regeneration: The rPOSTN-loaded ACF scaffold led to significant improvements in tendon regeneration, with features becoming comparable to normal Achilles tendons by the 8-week postoperative mark.
-
Improved Mechanical Properties: The regenerated tendons with the ACF-rP method showed superior mechanical properties, including a higher failure force and an elastic modulus near the value for native tendons.
-
Structural Similarities to Native Tendons: The rPOSTN delivery produced a collagen ultrastructure with a native periodic crimp pattern and a higher Young’s modulus, suggesting that rPOSTN is key for regenerating tendons with mechanical properties similar to native ones.
-
Reduced Bone Loss: The study also demonstrated that bone loss at the tendon insertion point was markedly reduced in the ACF and ACF-rP groups, with the most significant bone quality improvement observed in the ACF-rP group.
-
Long-term Healing Outcomes: Evaluations at 12 weeks postoperatively further confirmed the superior healing outcomes due to rPOSTN delivery. The collagen bundles were more mature and orderly aligned, suggesting an improvement in tendon functions.
-
Transcriptional Profiling of Tendon Development: Profiling the transcriptional aspects of tendon development processes can help identify key molecules that could potentially promote tendon regeneration. This could provide valuable insights into how tendon stem/progenitor cells (TSPCs) work and how they could be better harnessed for healing.
-
Use of Periostin (Postn): Periostin, an extracellular matrix-associated protein, has been found to contribute to the maintenance of TSPC functionality and promotes tendon regeneration. Utilizing periostin, particularly in the form of recombinant periostin (rPOSTN), could be a viable solution for promoting the proliferation and stemness of TSPCs, and maintaining their tenogenic potentials.
-
Long-Term In Vitro Passage with rPOSTN: The utilization of rPOSTN for TSPCs during long-term in vitro culture can be a practical approach to protecting TSPCs against functional impairment.
-
Biomimetic Parallel-Aligned Collagen Scaffold: Construction of a biomimetic parallel-aligned collagen scaffold could be another practical approach to facilitate TSPC tenogenesis. This scaffold, particularly when loaded with rPOSTN, can promote endogenous TSPC recruitment and tendon regeneration.
-
Growth Factors Application: The use of growth factors, such as transforming growth factor beta (TGF-β), insulin-like growth factor 1 (IGF-1), platelet-derived growth factor (PDGF), and growth differentiation factor 5 (GDF5), can promote tendon injury healing.
-
Hypoxia, Three-Dimensional Culture, and Substrate Patterning: Creating conditions that mimic the native environment of TSPCs, such as inducing hypoxia, three-dimensional culture, and substrate patterning, can potentially enhance their growth and functionality.
-
Combination of Biomimetic Scaffold and rPOSTN: Using a biomimetic scaffold in combination with rPOSTN, particularly in in vivo models such as a rat full-cut Achilles tendon defect model, can achieve structural and functional regeneration of tendons.
The Functions and Mechanisms of Low-Level Laser Therapy in Tendon Repair (Review)
Introduction
- Tendon injuries, often caused by overuse, result in structural changes and various adverse reactions, such as swelling and irregular collagen arrangement.
- Treatments for tendon injuries include conservative treatments, surgery, and specific exercises; among conservative treatments, LLLT has shown promising results.
- LLLT, or photobiomodulation, reduces the severity of tendon injuries by locally applying short-wavelength monochromatic light.
LLLT in Clinical Practice
- Among non-surgical treatments, injection therapy, including MSCs, is highly respected.
- Adipose-derived mesenchymal cells (ASCs) are considered optimal for inclusion in non-surgical protocols as they promote tendon repair more effectively than bone marrow MSCs (BMSCS).
LLLT Mechanism: C Cytchrome C Oxidase
- The effectiveness of LLLT is closely related to photon absorption and cytochrome C oxidase (CCO).
- LLLT promotes ATP synthesis and respiratory rate, which aid in tendon injury repair.
- In case of failure in CCO activation, laser therapy does not yield desired outcomes.
LLLT in the Three Phases of Tendon Repair
- The process of tendon healing is divided into three main phases: the inflammatory phase, the cell proliferation phase, and the tendon shaping (remodeling) phase.
- LLLT has distinct mechanisms of action in each phase of tendon repair.
LLLT Promotes Angiogenesis During the Inflammatory Phase
- In the inflammatory phase, LLLT primarily promotes angiogenesis.
- The condition for LLLT to promote angiogenesis is hypoxia; LLLT can regulate the activity of angiogenic factors.
- The process is mainly associated with hypoxia-inducible factor 1α (HIF-1α) activation, a large expression of VEGF, and downregulation of matrix metallopeptidase 2 (MMP-2).
- LLLT helps to improve angiogenesis and tendon injury recovery.
Note of Caution
- While LLLT can lead to improved tendon recovery, it may cause excessive upregulation of some growth factors in the final phase of tendon repair, which can result in tendon fibrosis.
LLLT Promotes the Synthesis of Collagen During the Cell Proliferation Phase
- LLLT and Fibroblast Proliferation: LLLT promotes collagen synthesis by enhancing fibroblast proliferation, thereby improving tendon healing. It does this by primarily interacting with a multifunctional growth factor - transforming growth factor-β (TGF-β).
- Role of TGF-β: TGF-β aids in wound healing and scar formation during tendon repair and plays a key role in muscle fibrosis by affecting changes in ECM-degrading proteases. It also reduces the number of senescent cells.
- LLLT and TGF-β Content: LLLT can reduce TGF-β content after a tendon injury, thereby decreasing the probability of tendon fibrosis and indirectly promoting collagen synthesis.
- DDR2 and MMP-2: LLLT enhances the gene expression of MMP-2, an important molecule in regulating collagen protein synthesis. This improves the chances of interaction with DDR2 collagen receptors, which regulates fibroblast proliferation and promotes ECM synthesis.
- Collagen Fibrils and Fibers: Following fibroblast proliferation, a large amount of collagen can be synthesized into collagen fibrils. These fibrils are bundled into collagen fibers, which greatly improve the mechanical strength of the tendon and the tension resistance of the injured tissue.
LLLT Reduces Inflammatory Response During the Tendon Shaping Phase
- Role of Cytokines: Tendon injury is associated with cytokines like tumor necrosis factor-α (TNF-α), Interleukin-1β (IL-1β), and Interleukin-10 (IL-10).
- LLLT and Cytokines: LLLT can effectively reduce the content of pro-inflammatory cytokines and regulate the mRNA expression of anti-inflammatory cytokines.
- LLLT’s Anti-inflammatory Effects: By decreasing the expression of the NF-kB gene, reducing COX-2 activity, and lowering the number of inflammatory mediators and pro-inflammatory factors, LLLT can produce anti-inflammatory effects and promote tendon repair.
- LLLT’s Effect on TNF-α and IL-6: LLLT has a significant effect on reducing the expression of TNF-α and IL-6 mRNA, which reduces tendon fibrosis and stiffness, maximizes fibroblast growth, and improves muscle contractility after tendon injury.
Conclusion and Perspectives
- Positive Effects of LLLT: LLLT has been found to have a positive effect on tendon repair, especially in terms of anti-inflammation and analgesia. The effectiveness of the treatment depends on the parameters used.
- Non-invasive and Non-thermal: LLLT is a non-invasive method that does not overheat the tissue. It utilizes the body’s heat to drive some of the materials to emit infrared to the damaged area.
- LLLT and Mitochondria: LLLT primarily affects the activity of mitochondria in cells, which leads to an increase in ATP content, a change in ROS species, and the expression of biological factor mRNA to stimulate tendon healing.
- Combination with Exercise Therapy: The use of LLLT, combined with certain exercise therapies, has been found to treat tendinopathy more effectively than other existing therapies.
- Mechanisms of LLLT in Tendon Repair: The mechanisms through which LLLT promotes tendon injury repair involve reducing the production of inflammatory factors, accelerating the release of anti-inflammatory factors, promoting angiogenesis, and promoting collagen synthesis.
Source:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8886125/
- PDGF-BB:
This is an extensive, well-researched, and organized literature review that summarizes the role of Platelet-Derived Growth Factor BB (PDGF-BB) in tendon healing. The main points I gathered from the passage are:
-
PDGF-BB influences and accelerates tendon healing via several mechanisms: controlling inflammation, promoting angiogenesis (formation of new blood vessels), stimulating cell proliferation and collagen synthesis, and inducing cell differentiation.
-
PDGF-BB impacts the early acute inflammatory responses, aids in the recruitment and activation of immune cells for pathogen destruction, and assists in the scavenging of dead or dying cells.
-
PDGF-BB promotes angiogenesis which provides better nutrient flow and elimination of metabolic waste products to enhance tendon healing. This is facilitated through the regulation of specific integrins such as αvβ3 and the signaling system PDGF-BB-PDGFR-β.
-
PDGF-BB promotes tendon cell proliferation and collagen synthesis. This effect is mediated through various signaling pathways including PI3K/AKT, ERK/AP-1, and HIF-1a/VEGF.
-
PDGF-BB induces cell differentiation via the Src/JAK2 and PDGFR signaling pathways. It can stimulate the transformation of stem cells into a tendon cell phenotype, promoting tendon regeneration.
-
The concentration of PDGF-BB affects its functions. Higher concentrations are useful for promoting cell proliferation, while lower concentrations enhance type I collagen synthesis.
-
Synergistic effects of multiple growth factors tend to produce better results for tendon healing. For example, the combination of PDGF-BB and GDF-6 or BMP-2 could provide more pronounced effects.
-
The optimization of growth factor delivery strategies, including novel drug delivery devices, is crucial for the controlled release of growth factors at the wound site. This can facilitate a positive healing outcome.
-
Further exploration of the precise mechanisms of PDGF-BB in tendon healing is needed to better understand its potential therapeutic role.
-
PDGF-BB for Cell Proliferation and Collagen Synthesis: Platelet-Derived Growth Factor-BB (PDGF-BB) stimulates tendon cell proliferation and aids in matrix remodeling by encouraging type I collagen synthesis. This response is dose-dependent and is influenced by the medium in which the cells are grown. However, the optimal concentration for promoting cell proliferation and collagen synthesis varies: higher concentrations are used for cell proliferation during early healing stages, and lower concentrations enhance the synthesis of type I collagen.
-
PDGF-BB for Differentiation of ASCs to Tendon Cells: PDGF-BB can induce the differentiation of adipose-derived stem cells (ASCs) into tendon cells, thereby facilitating tendon tissue regeneration.
-
Combination of PDGF-BB and GDF-6 for Tenogenic Differentiation of ASCs: The combined use of PDGF-BB and Growth Differentiation Factor 6 (GDF-6) significantly enhances the induction of tenogenic differentiation of ASCs, indicating that synergistic effects of multiple growth factors tend to yield better results.
-
PDGF-BB and BMP-2 for Tendon Differentiation of Stem Cells: In a rat patellar tendon avulsion model, it was found that PDGF-BB and Bone Morphogenetic Protein-2 (BMP-2) could induce tendon differentiation of stem cells when directly immobilized on heparin-bound polycaprolactone (PCL)/Pluronic F127 membranes, resulting in accelerated regeneration.
-
Sustained Release of Growth Factors: A sustained release of growth factors, such as PDGF-BB and BMP-2, maximizes their biological function in vivo, maintaining their biological activity long enough for effective regeneration.
PDGF-BB is an important growth factor that has a significant role in tendon healing. Its impact is mediated through various mechanisms including controlling inflammation, promoting angiogenesis, stimulating cell proliferation and collagen synthesis, and inducing cell differentiation
https://ars.els-cdn.com/content/image/1-s2.0-S0169409X14002786-gr3_lrg.jpg
Fig. 3. Key molecular, cellular and matrix changes occurring during the three main phases of tendon repair. Each healing stage is characterized by involvement of different growth factors, activation of certain cell types and production of essential matrix proteins, which collectively contribute to the replacement of the initial fibrous tissue with more a tendonous regenerate. Based on [45], [46].
Source:
https://www.sciencedirect.com/science/article/pii/S0169409X14002786
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9599567/
- Human induced pluripotent stem cells (hiPSCs)
Using hiPSCs with Mohawk (Mkx), we could produce artificial tendon tissue." explains Hiroki Tsutsumi, lead author of the study.
- The researchers used hiPSCs and introduced the Mohawk (Mkx) transcription factor to drive the differentiation of stem cells into tendon cells.
- In a mouse model of tendon rupture, the implanted artificial tendons integrated well with the surrounding tissue and recruited tendon cells from the host.
Source:
https://www.sciencedaily.com/releases/2022/02/220222121305.htm
- Nitric Oxide Therapy
Possible Nitric Oxide enhancement solution for tendon/ligament regeneration.
-
Nitric Oxide (NO) Therapy for Angiogenesis: NO therapy is emerging as a promising treatment for inducing the regeneration of injured tendons by promoting angiogenesis, the formation of new blood vessels. This is particularly crucial for tendons, which have limited blood supply, making regeneration difficult.
-
NO-Loaded Metal–Organic Frameworks (MOFs) Encapsulated in PCL/Gel Aligned Coaxial Scaffolds (NMPGA): These are designed to deliver NO in a controlled manner. The NO-loaded MOFs are encapsulated within Polycaprolactone (PCL)/Gelatin (Gel) aligned coaxial scaffolds. This system allows for a slow and steady release of NO over a period of 15 days, promoting angiogenesis, the maturation of collagen, and the recovery of biomechanical strength of the regenerated tendon tissue.
-
Copper-Based MOFs for NO Storage: Copper-based MOFs, particularly HKUST-1 (HK), provide an effective means of storing and releasing NO due to their high porosity, large specific surface area, and an abundance of active sites. The copper ions within these MOFs also promote angiogenesis.
-
NO-loading through 4-(Methylamino) Pyridine (4-map) Modification: This modification technique enhances the payload of NO in the MOFs, thus increasing the therapeutic potential of the system.
-
PCL/Gel Aligned Scaffolds for Enhanced Biomechanical Strength: These scaffolds mimic the organized native collagen fibers in tendons, enhancing the biomechanical strength of regenerated tendons.
-
HK Loaded with NO (NMHK) Encapsulated into the PCL Cores: This design separates the particles from external water, preventing undesired NO release and promoting slow, steady release of NO, ideal for tendon regeneration.
-
NO-based Therapy for Future Biomedical Application: This study provides a promising foundation for the development of NO-based therapy, which could be applied to other areas in biomedicine.
Source:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8187533/
- Silk Protien
Repairing tendons with silk proteins
- During the body’s natural healing processes, tendon and other cells are recruited to reconstruct the tendon’s original matrix of aligned connective tissue fibers. But this reconstruction can take weeks to months and the resultant tendon is often imperfect. This results in weakness, chronic pain and decreased quality of life. Possible treatments for tendon injuries include tendon tissue grafts from patients or donors, but these pose risks such as infections, transplant rejection or necrosis. Synthetic transplants have been attempted, but mechanical, biocompatibility and biodegradation issues have hampered these efforts. Another approach is to use mesenchymal stem cells (MSCs), specialized cells that play a pivotal role in tissue regeneration. At the wound site, they can differentiate into various cells types and produce signaling molecules which regulate immune response, cellular migration, and new blood vessel formation; this enables tissue regeneration.
Source:
https://www.sciencedaily.com/releases/2022/05/220503091508.htm
- Tendon Stem/Progenitor Cells (TSPCs)
Research suggests Tendon Stem cells are renewing and regenerative.
-
Understanding Tendon Stem/Progenitor Cells (TSPCs): These cells are vital for replenishing tendon cells by undergoing self-renewal and differentiation. A deeper understanding of their biological properties could help identify better treatment options for tendon injuries.
-
Understanding the Hierarchical Tendon Structure: This structure, optimized for specific functions, transforms mechanical loads into biochemical signals. If we can better understand how these signals regulate tendon metabolism and structural properties, we may develop better interventions for tendon injuries.
-
Enhancing the Natural Healing Process: The natural healing process occurs in three phases: inflammation, proliferation, and remodeling of the extracellular matrix (ECM). Interventions to enhance this process could improve tendon repair.
-
Addressing Chronic Tendon Injuries (Tendinopathy): Tendinopathy is generally thought to result from repetitive mechanical overloading, genetic predisposition, and age-related degeneration. Identifying effective therapies for this condition is a significant challenge in sports medicine and orthopedic surgery.
-
Overcoming the Limitations of Natural Tendon Healing: Tendon healing often results in scar tissue formation, characterized by disorganized collagen matrix and increased non-collagenous ECM. Strategies to overcome these limitations could significantly improve outcomes for patients with tendon injuries.
-
Improving Current Treatments: Existing treatments, including NSAIDS, corticosteroid and PRP injections, exercise-based physical therapy, and surgery, often have limited effectiveness, particularly for tendinopathy. There’s a need to develop new treatments, possibly based on a more in-depth understanding of tendon biology.
-
Validating Heavy Slow Resistance (HSR) Training: This type of training has shown promise in reducing pain and improving collagen fibril morphology in a small number of patients, but its effectiveness needs to be verified with large randomized controlled trials.
-
Increasing Understanding of Cellular and Molecular Mechanisms of Tendinopathy: Current therapeutic strategies are mainly palliative due to the limited understanding of these mechanisms. In-depth research could lead to the development of more effective treatment options.
Source:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6815631/
- Aim to scarless healing
-
Platelet Rich Plasma (PRP): PRP contains a multitude of growth factors that have roles in cell recruitment, proliferation, and angiogenesis, making it potentially useful in the treatment of tendinopathies such as lateral epicondylitis and Achilles tendinitis. In addition, the use of platelet rich fibrin matrix might provide a slower, more controlled release of factors as the fibrin matrix absorbs, which could be a key for successful treatment.
-
Animal Models for Studying Scarless Healing: Various animal models can provide insight into scarless healing process and may allow for the development of treatments that mimic this process in humans. The examples include:
-
Fetal Sheep Model: This model can demonstrate the differences in healing responses between adults and fetuses. It might help us understand the processes that lead to scarless healing in fetuses, as opposed to scar-mediated healing in adults.
-
MRL Mouse Model: The MRL mouse possesses the ability to heal via tissue regeneration in the post-natal setting. Studying this model may provide insights into scarless healing.
-
PU-1 Null Mouse Model: This model is genetically incapable of mounting a standard inflammatory response following injury because it lacks macrophages and functioning neutrophils. These mice can repair skin wounds in a scar-free manner, thus providing a unique opportunity to study scarless healing.
-
Axolotl Model: Axolotls are salamanders with extraordinary healing and regenerative capacities. Studying these animals might shed light on the mechanisms of scarless healing.
-
Fetal Mouse Skin Xenografts on Fertilized Chicken Eggs: This model might help in understanding the process of fetal wound healing.
-
Heart Infarction Model in Zebrafish: This model demonstrates scarless regeneration following heart infarction. Studying this process might provide valuable insights into scarless healing in human tissues.
-
source:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6084432/
Other Sources:
Functional regeneration and repair of tendons using biomimetic scaffolds loaded with recombinant periostin | Nature Communications
More research:
Repairing tendons with silk proteins
During the body’s natural healing processes, tendon and other cells are recruited to reconstruct the tendon’s original matrix of aligned connective tissue fibers. But this reconstruction can take weeks to months and the resultant tendon is often imperfect. This results in weakness, chronic pain and decreased quality of life.
Possible treatments for tendon injuries include tendon tissue grafts from patients or donors, but these pose risks such as infections, transplant rejection or necrosis. Synthetic transplants have been attempted, but mechanical, biocompatibility and biodegradation issues have hampered these efforts.
Another approach is to use mesenchymal stem cells (MSCs), specialized cells that play a pivotal role in tissue regeneration. At the wound site, they can differentiate into various cells types and produce signaling molecules which regulate immune response, cellular migration, and new blood vessel formation; this enables tissue regeneration.
Source:
Repairing tendons with silk proteins – ScienceDaily
https://forum.enlightenedstates.com/t/tendon-ligament-repair/65167/25
The tendon healing process is complexly orchestrated by a variety of secreted molecules [42]. Initially, certain inflammatory cytokines, such as interleukin (IL)-6 and IL-1β, are produced by the invading inflammatory cells. Later, tissue repair is facilitated by a number of growth factors, which are released by cells located at the injury site. bFGF (basic fibroblast growth factor), BMPs (bone morphogenetic proteins)-12, -13, and -14 also known as GDFs (growth and differentiation factors) -5, -6 and -7 respectively, TGFβ (transforming growth factor beta), IGF-1 (insulin-like growth factor-1), PDGF (platelet-derived growth factor) and VEGF (vascular endothelial growth factor) are involved in different phases of the healing process with diverse molecular effects (Fig. 3). During the repair process, tendon cells are activated and both synthesize and degrade ECM components, thereby participating in the slow, continuous process of tendon remodeling [39], [43], [44].
Two cellular mechanisms of tendon healing, known as extrinsic and intrinsic healing, have been suggested [41], [45]. It is now believed that these two mechanisms normally act cooperatively. The hypothesis is that first fibroblasts and inflammatory cells from the tendon periphery, blood vessels and circulation are attracted to the injured site contributing to cell infiltration and the formation of adhesions. Thereafter, intrinsic cells from the endotenon are activated as they migrate and proliferate at the injury site, reorganizing the ECM and giving support to the internal vascular networking [38], [46]. The origin of the reparative cells remains in debate. In 2007, an enlightening study from Kajikawa et al., used a model of tendon injury applied to two different chimeric rats, one expressing green fluorescent protein (GFP) in circulating mesenchymal cells, and the other in the patellar tendon. The data were consistent with the biphasic pattern of tendon healing. This comprises an initial invasion of circulating MSCs followed by the activation of local cells, which participate in the proliferative phase and carry out the long remodeling phase [45].
In most patients, especially aged individuals, the healed tendon usually does not regain the mechanical properties of the uninjured tissue. The reduced strength of the repaired tissue compared to the native tendon results from reduced integration of collagen fibers with a higher ratio of collagen type III to collagen type I. As a consequence, the tendon thickens and stiffens to overcome the lower unit mechanical strength; thus the tendon quality and its functional activity are inferior to that of healthy tendon.
2.1. Growth factors
Tendon injury stimulates the production of a variety of growth factors at multiple stages in the healing process [40], [42] leading to increased cellularity and tissue volume [47]. Increased expression of growth factors is particularly prominent in the early phases of healing [48], [49]. The following growth factors are important in tendon healing: bFGF, BMP-12, -13, -14, CTGF (connective tissue growth factor), IGF-1, PDGF, TGFβ, and VEGF [49], [50], [51], [52]. In the following section these factors are briefly introduced before describing in vitro and in vivo experiments investigating the role of the factors in tendon healing (Table 1). No human study investigating recombinant growth factors in tendon healing has been published in the literature.
2.1.1. bFGF
Chang et al., found upregulated bFGF mRNA in mature tenocytes and in fibroblasts and inflammatory cells surrounding the healing site in the tendon sheath [53]. Being elevated early in the healing process [48], [49], bFGF is well positioned to promote the early events in tendon healing [54].
2.1.2. BMP
BMP-12, -13, and -14, also known as GDF-7, -6, and -5 respectively, stimulate mitogenesis, and are established tenogenic factors with the potential of driving differentiation of MSC in vitro [55] and in vivo [56]. BMPs are elevated early in the tendon healing process, gradually decreasing thereafter [48], [49]. BMP-2 plays a role at the enthesis, the anatomical junction of tendon and ligament to bone. New bone formation can be induced by BMP-2 within a tendon with comparable characteristics to the enthesis. However, in intratendinous healing this bone formation is clearly undesirable [57], [58], [59].
2.1.3. CTGF
In contrast to the previously described factors, CTGF exhibits a sustained increase in gene expression persisting over 21 days during healing of chicken flexor tendons [50]. In the rat supraspinatus injury model of Würgler-Hauri et al., CTGF was moderately expressed in both the insertion and midsubstance area throughout all time points [49].
2.1.4. IGF-I
IGF-1 induces tenocyte migration and increases synthesis of the ECM, including collagen [60]. Elevated IGF-1 mRNA and protein expression levels were found in healing rabbit ligaments 3 weeks after injury and in healing equine tendons after 4 to 8 weeks [61], [62]. IGF-1 seems to be particularly important during the formation and remodeling stages of healing.
2.1.5. PDGF
Increased PDGF-levels have been found in healing tendons [63]. Elevated expression of the PDGF receptor β was found by Chan et al., to persist for over 6 months after tendon injury, potentially indicating the important role of PDGF during the entire tendon repair period [64].
2.1.6. TGFβ
Besides tendon cell migration and mitogenesis, TGFβ especially stimulates production of the ECM, including increases in the production of collagen types I and III by all the 3 isoforms TGFβ1, TGFβ2, and TGFβ3 [65]. High levels of expression and activity of TGFβ are found throughout the course of tendon-healing [66], [67]. Resident tenocytes and infiltrating cells from the surrounding tendon sheath show increased expression of TGFβ1 mRNA [68]. Correspondingly, TGFβ1/3 receptor (CD 105; endoglin) expression was also found to be upregulated at the repair site [69]. Juneja et al., found a biphasic pattern of TGFβ expression corresponding to an early peak of TGFβ1 and a late peak of TGFβ3 expression during healing [70]. Heisterbach et al., also found early and late peaks of TGFβ1 expression [48]. However, there are also data indicating that TGFβ1 provokes increased fibrotic scar formation resulting in tendon adhesions [71], [72]. In a rabbit model adhesions were reduced using an anti-TGFβ1 antibody, but were not further influenced by the addition of an antibody against the isoform TGFβ2 [66]. Possibly an imbalance between the TGFβ1-induced ECM-formation and tendon remodeling is responsible for the formation of adhesions [73], [74]. Thus, defining the appropriate doses and combinations of isoforms could be essential for the successful application of TGFβ in tendon healing.
2.1.7. VEGF
Angiogenesis is important in both tendon degeneration, in cases of impaired blood supply, and in regeneration, for which the best possible capillary permeability is desirable [41]. VEGF promotes angiogenesis in tendon healing [75], and its activity rises after the inflammatory phase, especially during the proliferative and remodeling phases. In a canine model of tendon transection, VEGF mRNA peaked 10 days after surgery [76].
Biologics for tendon repair - ScienceDirect
Stage one (Inflammatory)
Platelets
Neutrophils
Monocytes
Erythrocytes
Circulation-derived mesenchymal stem cells
Stage two (Reparative, Proliferation)
Cellularity and matrix production
Collagen Type III (increases)
Activation of Local Tendon
Stem/progenitor Cells
Stage three (Remodeling)
Cellularity matrix production (declines)
Collagen type III (declines)
Collagen type I (increases)
some more information:
-
Tendon Development: Earlier studies on tendon development were limited due to the lack of a tendon-specific lineage marker. However, recent research identified several markers selectively associated with tendon and musculoskeletal tissues, helping trace the formation and maturation of tendons.
-
Scleraxis (Scx): Scx, originally discovered as a sclerotome marker, is expressed in tendon progenitor cells and mature tenocytes. Scx is found in all muscle-to-bone attachment sites in chick embryos.
-
Scx/E47 Heterodimer: Scx binds a short cis-acting element, the tendon-specific element 2 (TSE2), to form the Scx/E47 heterodimer. This heterodimer activates the collagen type Iα1 (COLI a1) proximal promoter.
-
Tenomodulin (Tnmd): Overexpression of Scx in tendon fibroblasts upregulates the expression of the tenomodulin (Tnmd) gene. Tnmd encodes a transmembrane protein selectively expressed in tendons and ligaments and is considered a late marker of tendon formation.
-
Origin of Tendon Progenitors: In situ hybridization analysis of the somitic mesoderm, which forms along the anterior-posterior axis of developing embryos in segmented animals, offers insights into the origin of tendon progenitors.
-
Scx-Expressing Progenitor Cells: These cells first appear between the myotome and sclerotome. They share a similar somitic domain with cells expressing Pax1, an early sclerotome marker. However, Pax1 expression is more ventromedial, while Scx is limited to cells nearest to the myotome.
-
Scx and MyoD Expression: Scx expression does not overlap with MyoD expression in the myotome. Instead, Scx-expressing progenitor cells are seen immediately adjacent to MyoD-positive cells. They form a fourth somitic compartment, syndetome, which is associated with the myotome and sclerotome.
-
Chick-Quail Chimera Model: This model was used to further investigate the origin of Scx-expressing tendon progenitors. When quail sclerotomal cells were implanted into chick embryos, these quail cells were capable of generating Scx-expressing progenitors.
-
Transplanted Quail Dermamyotomes: In contrast, when quail dermamyotomes were transplanted into embryos, the quail cells did not contribute to the formation of Scx-expressing progenitors.
-
Development of Muscle-Tendon-Bone Complex: Despite occupying distinct spatial regions, the generation of Scx-expressing progenitors requires signals from both the sclerotome and myotome.
-
Role of Pax1: Overexpression of Pax1 in the sclerotome prevented the induction of Scx, suggesting that Pax1-positive sclerotome can’t generate tendon progenitors.
-
Role of Myotome: The myotome appears to be vital for Scx induction, as evidenced by the fact that no Scx induction was observed in somites where the dermamyotome had been removed before myotome formation.
-
Fibroblast Growth Factor 8 (FGF8): Secreted by the myotome, FGF8 is partly responsible for inducing Scx expression through the Ets transcription factors Pea3 and Erm.
-
Downregulation of Pax1: FGF8 also functions to downregulate Pax1 in Scx-positive domains in the sclerotome. However, this repression alone does not result in Scx expression.
-
Other FGF Family Members: Other FGF family members, such as FGF4, also positively regulate the induction of tendon progenitors.
-
FGF4 Transcripts: These transcripts are located at the extremities of muscles, near the attachment sites of tendons in the embryonic chick wing.
-
Overexpression of FGF4: The overexpression of FGF4 induces ectopic expression of Scx and tenascin in wing buds.
-
Transforming Growth Factor-β (TGFβ) Superfamily: Members of this family are involved in tendon development regulation. TGFβ-2/3 ligand and its receptors were detected throughout the tertiary bundles in the tendon midsubstance and endotenon during the intermediate stages of tendon development in the chick embryo. In mouse patellar tendon development, all cells in the tendon responded to TGFβ and BMP signaling at all stages examined, including embryonic and postnatal periods.
-
In Vitro Micromass Culture of Chick Mesodermal Cells: Significant upregulation of tendon markers Scx and Tnmd was observed when cultured with TGFβ, accompanied by a reduction in cartilage markers. This was mediated by the canonical Smad signaling pathway.
-
Disruption of TGFβ Signaling: Disruption in a Tgfb2−/− Tgfb3−/− mouse model resulted in the loss of most tendons and ligaments.
-
Growth and Differentiation Factors (GDF): Members of the BMP family, these are additional regulators of tendon development. Mice deficient in GDF-5 demonstrated inferior matrix composition and mechanical strength in their Achilles tendons, whereas null mutation of GDF-6 in mice resulted in substantially lower levels of tail tendon collagen content. GDF-7 deficiency didn’t affect biochemical composition of mouse tail tendon fascicles, suggesting differential effects of GDF isoforms in promoting tendon development.
-
Collagenous Fibrils Development: Tendon progenitors lay down small diameter collagenous fibrils during embryonic development, a process which continues after birth.
-
Fibrillar Collagens: These collagens constitute the basic structural framework of tendons and ligaments and are synthesized in a precursor form, procollagen, which once secreted into the extracellular space and modified, self-assemble into cross-striated fibrils, yielding collagen fibrils.
-
Collagen Fibers: Fibers bundled into which tenocytes reside and maintain the extracellular matrix. The number and diameter of collagen fibers vary among species.
-
Endotenon, Epitenon, and Paratenon: These are layers of connective tissue that wrap and form the higher structural units called fascicles, forming the tendon. The paratenon functions as an elastic sleeve, permitting free movement of the tendon against surrounding tissues.
-
Collagen Type I: This is the predominant fibril-forming collagen in tendons and is formed as a heterotrimer consisting of two α1 chains and one α2 chain. It co-polymerizes with collagen type V, which regulates collagen fibrillar structure.
-
Non-fibrillar Collagens: These include the fibril-associated collagens with interrupted triple helices (FACIT) such as collagens type XII and XIV. They serve a regulatory role during collagen fibrillogenesis.
-
Collagen Type XII: This type of collagen is believed to integrate adjacent matrix components due to its ability to bind proteoglycans, fibromodulin, and decorin, while also interacting with collagen type I fibrils.
-
Collagen Type XIV and XII: Collagen type XIV plays a role in integrating fibrils into fibers during development, whereas collagen type XII assumes the same structural and functional role in the mature tendon.
-
Small Leucine-rich Proteoglycans: These, including decorin, biglycan, fibromodulin, and lumican, play a role in organizing fibril assembly and the resulting ultrastructure of the tendon.
-
Decorin-deficient Mice: They develop structurally impaired tendons with abnormal, irregular fibril contours. In these mice, biglycan expression significantly increases, suggesting a functional compensation.
-
Fibromodulin and Lumican: Deficiency in fibromodulin leads to a significant reduction in tendon stiffness, which is further decreased when combined with lumican deficiency. Fibromodulin might be required to stabilize small-diameter fibrils during early tendon development, with lumican taking on this role later.
-
Biglycan and Fibromodulin: These proteins are also found to maintain the niche of tendon stem cells. Tendon stem cells from biglycan- and fibromodulin-deficient mice show decreased expression of the tendon marker Scx and collagen type I, compared to cells from wild-type mice.
-
Glycoproteins: Important constituents of tendon. Tenomodulin (TNMD), a type II transmembrane glycoprotein identified as a marker of mature tenocytes, is positively regulated by Scleraxis. Loss of TNMD expression in gene-targeted mice leads to reduced tenocyte proliferation and density, and variable diameters of collagen fibrils.
-
Tenascin C (TNC): Before the discovery of Scx, TNC, a member of a family of four ECM glycoproteins in vertebrates, was used as the primary tendon marker. It’s an ECM component directly regulated by mechanical stress. The degree of tenascin C deposition is greatest in areas of high mechanical loading.
-
Covalent Cross-Links: As tendons mature, covalent cross-links are formed between collagen fibrils at the overlapping ends of adjacent collagen molecules.
-
Correlation with Elastic Modulus: Collagen cross-links significantly correlate with the increasing elastic modulus of in utero tendon, whereas correlations between mechanical properties and collagen, glycosaminoglycan, and dsDNA content are weak.
-
Enzyme Lysyl Oxidase: The formation of collagen type I cross-links in tendons is primarily driven by this enzyme. It acts on specific lysine and hydroxylysine residues and results in stable trivalent cross-links, enhancing collagen interconnectivity and fibril stability.
-
Age-related Increase: Mechanical properties, collagen cross-links, average fibril diameter, and the distribution of fibril diameter have all been found to increase with age. However, the structure-function relationship between these biochemical characteristics and tendon mechanical properties is still unclear.
-
Tendon Adaptation to Mechanical Stresses: Extensive research has been conducted on how tendons adapt to mechanical stresses. The majority of these studies focus on the tendon proper, demonstrating that the type of loading dictates the balance between anabolic and catabolic pathways in resident fibroblasts.
-
Role of Mechanical Stimulation in Tendon Healing: The importance of mechanical stimulation in the healing process of tendons has been acknowledged and is discussed in further detail below.
-
Effects of Reduced Muscle Loading: In the aforementioned mouse model, mice with reduced muscle loading showed less mineral deposition, impaired fibrochondrocyte and matrix organization, and inferior mechanical properties at later time points, all indicating the importance of adequate mechanical loading.
-
Physical Rehabilitation Protocols Post-Injury: Following a tendon injury, implementing physical rehabilitation protocols is critical for regaining tendon function.
-
Three Overlapping Stages of Tendon Healing: The healing response of tendons is predictable and is generally divided into three overlapping stages: inflammation, proliferation/repair, and remodeling.
-
Inflammatory Stage: During the inflammation stage, a blood clot forms immediately after tendon vessel rupture. This clot triggers the release of chemoattractants and forms a scaffold for incoming cells. Inflammatory cells like neutrophils, monocytes, and lymphocytes migrate from surrounding tissues to the wound site where they engage in phagocytosis.
-
Proliferation/Repair Stage: Starting about two days after injury, the proliferation or repair stage begins. Here, fibroblasts from the paratenon or surrounding synovial sheath are drawn to the wound area where they proliferate. Intrinsic tenocytes also migrate to the injury site and begin to proliferate, synthesizing an extracellular matrix and establishing an internal neovascular network. During this stage, the matrix created by tenocytes is primarily composed of collagen type III.
-
Remodeling Stage: The remodeling phase starts 1-2 months after injury. This stage sees tenocytes and collagen fibers aligning in the direction of stress. More collagen type I is synthesized, and there is a decrease in cellularity and collagen type III and glycosaminoglycan contents.
-
Debate on Elements Impairing Tendon Regeneration: There’s an ongoing debate regarding which elements of the adult tissue hinder tendon regeneration.
-
Study by Bayer et al.: A study by Bayer et al. demonstrated embryonic-like fibrillogenesis by adult human tendon fibroblasts when cultured in a fibrin gel contracted around a suture. This suggests that the hormonal/mechanical milieu may inhibit the regenerative potential of adult tendons.
-
Study by Favata et al.: Conversely, another study by Favata et al. transplanted fetal tenocytes contracted around a suture into an adult environment and performed a partial tenotomy on the engineered tendon construct. Compared to adult fibroblasts, the fetal tenocytes filled the defect without scar. This indicates that the adult environment may not impede scarless tendon regeneration, but such dysfunctional healing might be inherent to aged cells.
-
Frequency of TSPCs Decreases with Age: Another recent study found that the frequency of tendon-derived stem/progenitor cells (TSPCs) decreases with age. Additionally, aged TSPCs show a decreased proliferation rate but an increased propensity for adipogenic differentiation.
-
Unclear Reason for Adult Tendon Regeneration Failure: Despite these studies, it remains unclear why adult tendons fail to regenerate. This uncertainty presents a clinical challenge for physicians and patients alike.
TENDON TISSUE ENGINEERING
Cells
- Cells in Tendon Tissue Engineering: Various types of cells are utilized in tendon tissue engineering, including tenocytes (tendon fibroblasts), dermal fibroblasts, and mesenchymal stem cells (MSCs). Tenocytes are known to synthesize components of the extracellular matrix, like collagen, proteoglycans, and glycoproteins, and significantly influence collagen fiber formation. When used in tendon repair models, tenocytes have demonstrated improved mechanical strength and matrix deposition.
- Limitations of Tenocyte Harvesting: However, there are certain drawbacks, such as potential secondary tendon defects at the donor site when harvesting autologous tenocytes. As an alternative, dermal fibroblasts are considered due to their accessibility and lower donor site morbidity. Studies have demonstrated their potential in tendon engineering, producing similar results to tenocyte-engineered tendons.
- Mesenchymal Stem Cells (MSCs) in Tendon Tissue Engineering: MSCs from various tissues, such as bone marrow, adipose, and tendon, offer a promising cell source for tendon tissue engineering, owing to their self-renewal and multi-lineage differentiation potential. For instance, Bone marrow MSCs (BMSCs) have demonstrated higher collagen production when used in certain tissue engineering models.
- Adipose-Derived Mesenchymal Stem Cells (ASCs): ASCs, which can be harvested through less invasive procedures and are available in greater quantities, have shown similar differentiation potential to BMSCs. When treated and placed under certain conditions, they have been able to form vascularized tendon-like structures.
- Tendon-Derived Stem Cells (TDSCs): Finally, TDSCs, a unique cell population in tendons with universal stem cell characteristics, have shown high regenerative potential. They have been used to promote superior tendon repair, but similar to tenocytes, their isolation might present donor site morbidities.
Scaffolds
- The Role of Scaffolds in Tendon Tissue Engineering: Scaffolds serve as critical elements in tendon tissue engineering. They provide biomechanical support to the healing tissue, allowing for endogenous cells to deposit native matrix and prevent re-rupture. Scaffolds can also facilitate improved tendon healing by promoting cell proliferation, aiding in matrix production, and organizing the matrix into functional tendon tissues.
- Modification of Scaffolds: To further improve tendon healing, scaffolds can be modified using various methods like cellular hybridization, surface modification, growth factor attachment, and mechanical stimulation-mediated cellular remodeling.
- Categories of Scaffolds: There are three main categories of scaffolds used in tendon tissue engineering:
- Native tendon matrices: These are derived directly from tendon tissues.
- Synthetic polymers: These are artificially made materials specifically designed for this purpose.
- Derivatives of naturally occurring proteins: These are created from proteins that naturally occur in the body, which are then modified for use as scaffolds.
Bioactive Molecules
- IGF-1: Insulin-like growth factor 1 (IGF-1) has shown promise in accelerating healing processes and enhancing the quality of repaired tissue. When applied to an inflamed rat Achilles tendon, IGF-1 was shown to mitigate inflammation-induced functional deficits. IGF-1 also stimulates fibroblast proliferation and migration at injury sites, increasing the production of collagen and other extracellular matrix structures during remodeling stages. For example, in a study, recombinant human IGF-1 stimulated the synthesis of proteoglycan, collagen, noncollagen protein, and DNA in rabbit flexor tendons.
- TGF-β: Transforming growth factor-beta (TGF-β) plays a role in matrix synthesis in tendon healing. It increases the production of collagen types I and III in rabbit tendon cells. It also drives tenocyte differentiation, indicating potential use in stem cell-based tendon tissue engineering. For instance, exposing equine embryo-derived stem cells (ESCs) to TGF-β led to an upregulation of scleraxis, a crucial gene for tenogenesis.
- GDF-5 (BMP-14): Growth differentiation factor-5, a member of the TGF-β family, has also been used successfully in tendon tissue engineering. For instance, GDF-5-treated rat adipose-derived stem cells expressed tendon-specific genes in both 2D and 3D electrospun matrix systems. The addition of these growth factors to a transected Achilles tendon improved its mechanical properties.
- bFGF: Basic fibroblast growth factor (bFGF) is another growth factor extensively used in tendon tissue engineering. It has shown a dose-dependent increase in proliferation and collagen type III expression in a rat patellar tendon injury model. A nanofibrous scaffold releasing bFGF was developed to promote bone marrow-derived stem cell proliferation and tenogenic differentiation.
- PDGF-BB: Platelet-derived growth factor BB (PDGF-BB) stimulated collagen and noncollagen protein production, as well as DNA synthesis in rabbit tendon in a dose-dependent manner. When delivered through fibrin glue, PDGF-BB improved the healing of rabbit medial collateral ligament.
Mechanical Stimulation
- Impact of Loading: Tendons play a vital role in transmitting force from muscles to bones. Therefore, their mechanical properties are extensively studied. Regarding tendon injury and repair, controlled mobilization of healing tendons is known to improve outcomes. However, the optimal timing and magnitude of loading is a subject of debate. Complete removal of load from healing tendons results in inferior mechanical properties, while too quick an introduction of exercise can also be detrimental.
- Cellular and Molecular Response: Recent studies have explored the cellular and molecular basis for tendon loading. When fibroblasts and mesenchymal stem cells are exposed to static or cyclic uniaxial tension, increased cellular proliferation, collagen production, and tenogenic gene expression are observed. Mechanical stretch induces tenogenesis via integrin-dependent signaling and biochemical pathways such as TGF-β. Conversely, tendon overuse injuries are thought to result from upregulated expression of catabolic and inflammatory mediators. Therefore, a clinical strategy of controlled mobilization, where progressively greater frequencies and magnitudes of loads are applied, can promote tenogenesis of endogenous progenitor cells, increase extracellular matrix synthesis, and minimize inflammatory and catabolic responses.
- Preconditioning of Engineered Tissue: Mechanical stimulation can also be used to precondition tissue engineered constructs prior to implantation. This preconditioning improves in vivo outcomes by augmenting the mechanical properties of these constructs through cell-mediated remodeling of the extracellular matrix.
- In Vivo Mechanical Loading: The effects of mechanical loading on the integration and remodeling of tissue engineered constructs implanted in vivo are beginning to emerge. Studies have shown that the mechanical environment following implantation or injection of healing agents plays a significant role in promoting the integration and remodeling of these constructs. This finding suggests the necessity of in vivo mechanical loading for the successful application of regenerative medicine.
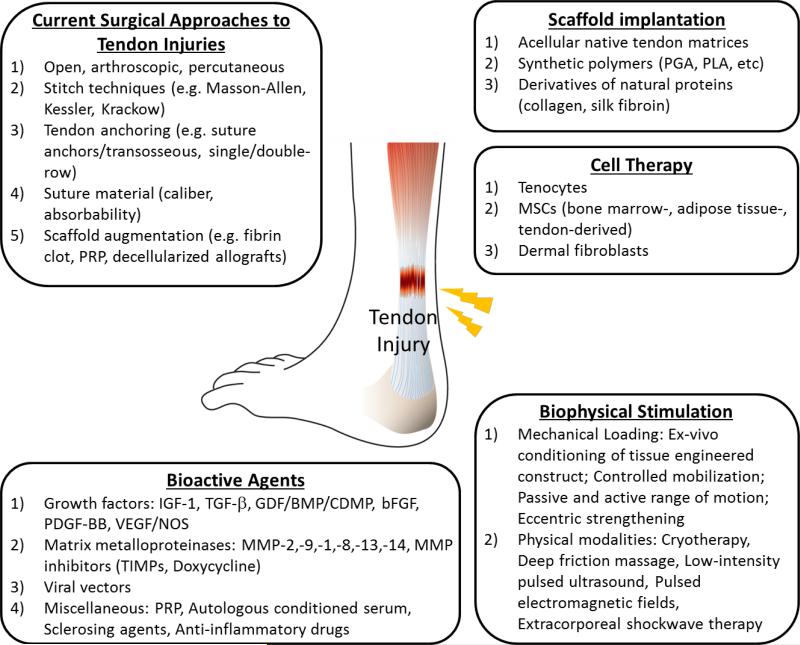
An overview of approaches for repair of tendon injuries. Briefly, surgical interventions and biophysical stimulation are currently employed in clinical care. Meanwhile, tissue engineering strategies are the cutting-edge of tendon healing and regeneration. Engineered replacement of injured tendon using a combination of cells, bioactive molecules, and scaffolds is under intensive investigation.
Source:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4041869/
Tendinitis Genotype Report
- GDF5 Gene: This gene encodes a growth factor involved in bone and cartilage formation. Variants in GDF5 are associated with an increased risk of Achilles tendon tears.
| rs143383 | Genotype | Risk Level |
|---|---|---|
| A/A | higher risk of Achilles tendon (common genotype)[ref] | |
| A/G | less of a risk of Achilles tendon problems | |
| G/G | half the risk of Achilles tendon problems |
- COL1A1 gene: encodes part of the type 1 collagen protein
| rs1800012 | Genotype | Risk Level |
|---|---|---|
| C/C | typical | |
| A/C | typical risk for Achilles tendon tear | |
| A/A | significantly reduced risk of Achilles tendon tear |
- COL5A1 gene: encodes a collagen formation protein involved in tendon formation.
| rs12722 | Genotype | Risk Level |
|---|---|---|
| C/C | half the risk of Achilles tendinopathy (higher levels of COL5A1) | |
| C/T | higher risk of Achilles tendinopathy (lower levels of COL5A1); increased risk of tennis elbow | |
| T/T | higher risk of Achilles tendinopathy (lower levels of COL5A1); increased risk of tennis elbow |
- MIR608 gene: microRNA 608 interacts with COL5A1 in collagen formation
| rs4919510 | Genotype | Risk Level |
|---|---|---|
| C/C | typical | |
| C/G | associated with chronic Achilles tendinopathy | |
| G/G | associated with chronic Achilles tendinopathy |
Inflammation-related genetic variants linked to tendinopathy
- BMP4 gene: encodes bone morphogenetic protein 4, a growth factor in the TGF-beta family that plays a role in neurogenesis, vascular development, and bone development.
| rs2761884 | Genotype | Risk Level |
|---|---|---|
| G/G | typical | |
| G/T | increased risk of tendinopathies | |
| T/T | 2-fold increased risk of tendinopathies |
- FCRL3 gene: encodes an Fc receptor protein that plays a regulatory role in the immune system. Variants in this gene are linked to rheumatoid arthritis and other autoimmune diseases.
| rs7528684 | Genotype | Risk Level |
|---|---|---|
| A/A | typical | |
| A/G | increased risk of tendinopathies | |
| G/G | increased risk of tendinopathies |
- TNF gene: encodes the inflammatory cytokine TNF-alpha
| rs1800629 | Genotype | Risk Level |
|---|---|---|
| A/A | Higher TNF-alpha levels; increased risk of Achilles tendon problems and knee tendon problems | |
| A/G | somewhat higher TNF-alpha levels; increased risk of Achilles tendon problems and knee tendon problems | |
| G/G | typical, better response to high protein/low carb diet |
Matrix metalloproteinase gene variants linked to increased risk of tendinopathy:
- MMP13 gene
| rs2252070 | Genotype | Risk Level |
|---|---|---|
| T/T | common genotype; increased risk of posterior tibial tendon problems (flat foot)[ref] | |
| C/T | increased risk of posterior tibial tendon problems (flat foot) | |
| C/C | decreased |
risk of posterior tibial tendon problems (flat foot) |
- MMP1 gene: matrix metalloproteinase-1 is involved in remodeling collagen type I, III, and V.
| rs1144393 | Genotype | Risk Level |
|---|---|---|
| C/C | increased risk of posterior tibial tendon problems | |
| C/T | increased risk of posterior tibial tendon problems | |
| T/T | decreased risk of posterior tibial tendon problems |
- MMP3 gene: matrix metalloproteinase-3 is involved in the remodeling of collagen.
| rs650108 | Genotype | Risk Level |
|---|---|---|
| G/G | increased risk of tendinopathies (high-level athletes) | |
| A/G | typical risk | |
| A/A | typical risk |
| rs679620 | Genotype | Risk Level |
|---|---|---|
| T/T | increased risk of tendinopathies (high-level athletes) | |
| C/T | typical risk | |
| C/C | typical risk |