More
Here’s a truth about being human
No matter how sharp our minds are, memory fades—each moment we live slowly dissolves, leaving only traces behind.
Synaptic Weakening: Memory strength relies on the stability of synaptic connections. AMPAR receptors on the synapse surface are key to maintaining this strength. During forgetting, these receptors are removed, weakening the synapse and reducing the efficacy of neural communication.
Synaptic Pruning: Microglia cells remove synapses that are underused. This process eliminates weaker connections, reducing the overall number of synapses within a neural network.
Reversal of Long-Term Potentiation (LTP): Synapses that have undergone LTP to strengthen memory can reverse this process through long-term depression (LTD). Enzymes like phosphatases dephosphorylate proteins involved in synaptic strengthening, leading to the weakening of the synapse.
Engram Cell Decay: Memory-related neurons, known as engram cells, can degrade over time. Protein turnover within these neurons, including the reduction of stabilizing proteins like PKMζ, leads to memory trace weakening.
Epigenetic Changes: Memory consolidation relies on gene expression, controlled by histone acetylation. During forgetting, histone deacetylases (HDACs) reduce the transcription of memory-supporting genes, weakening synaptic stability.
Interference: New memories can form synaptic connections that interfere with or overwrite existing ones, making older memories less accessible.
Sleep and Synaptic Downscaling: During sleep, particularly REM and slow-wave sleep, the brain reduces the strength of certain synapses in a process known as synaptic downscaling, which decreases overall synaptic activity and can contribute to forgetting.
But that stuff is not cool, so we said to hell with this and decided to offer: Memorivium Pro
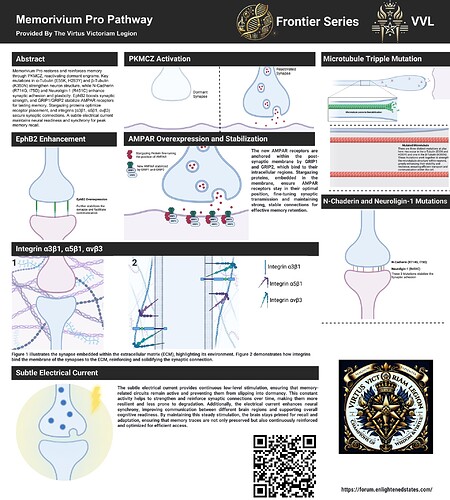
At the heart of this transformation is PKMCZ, a potent primer that sets the stage for the reactivation of dormant engrams, memory traces long buried beneath the layers of time. PKMCZ doesn’t just nudge forgotten memories to the surface—it restores them, breathing life into what was once thought lost. It acts as the catalyst, priming the brain for the next phase of enhancement, ensuring that these old memories are ready for reinforcement by the suite of cutting-edge mutations that follow.
The first layer of this enhancement begins with α-Tubulin (E55K, H283Y) and β-Tubulin (K350N), three key mutations that fortify the cytoskeleton of neurons. Tubulin is the backbone of the brain’s microtubules, the inner highways that transport vital materials within neurons. These mutations dramatically increase the structural integrity of these pathways, preventing the natural depolymerization that would otherwise weaken the neuron’s infrastructure. The result? Memory traces—both newly formed and restored by PKMCZ—are given a stable foundation to anchor into, ensuring they won’t degrade. The secondary benefit here is that, with enhanced microtubule stability, neuronal longevity improves. This means that neurons remain functional and resilient for far longer, reducing age-related cognitive decline, and increasing processing efficiency at every level of thought and cognition.
Next comes N-Cadherin (R714G and I75D), two mutations that amplify trans-synaptic adhesion, the very force that holds neurons together across synapses. N-Cadherin is a critical protein in establishing and maintaining strong neural connections—the very threads upon which memories are stored. By strengthening these connections, this mutation ensures that once memories are formed or reactivated, they are locked into place with far greater tenacity. What’s more, this heightened adhesion doesn’t just serve memory—it boosts overall cognitive performance, enhancing learning speed, problem-solving ability, and pattern recognition, as neural circuits become more robust and efficient at transferring information.
The Neuroligin-1 (R451C) mutation takes this even further by enhancing synapse formation and maintaining synaptic plasticity. Neuroligin-1 is responsible for organizing synaptic connections, ensuring that neurons communicate efficiently. This mutation supercharges its effect, making it easier for neurons to form new connections in response to learning, while simultaneously reinforcing existing pathways. In practical terms, this means that every experience, every piece of information, becomes deeply embedded into the brain’s network, with minimal risk of it fading away. The secondary benefit? A brain optimized by Neuroligin-1 mutations becomes hyper-adaptive, meaning it’s not just faster at learning, but also more flexible in adapting to new environments, skills, and stimuli.
As memories solidify, EphB2 mutation comes into play. EphB2 is a regulator of synaptic strength, making it essential for rapid and long-lasting changes in synapse connectivity, key to memory formation. Enhancing EphB2’s activity means that memory traces become nearly unbreakable once formed, but it also allows the brain to continuously refine these connections. This mutation enables the brain to strengthen and optimize its neural pathways during and after learning, making it easier to store vast amounts of data. The secondary advantage of EphB2 is its ability to enhance cognitive flexibility—the capacity to switch between tasks or integrate multiple streams of information at once, allowing for more creative thought and complex problem-solving.
For true memory retention, AMPAR receptors, stabilized by GRIP1 and GRIP2, take center stage. AMPAR is pivotal in the transmission of excitatory signals between neurons, facilitating the communication that underpins long-term potentiation (LTP)—the very basis of long-lasting memory. By anchoring AMPAR receptors in place, GRIP1 and GRIP2 ensure that synaptic signals remain strong and consistent, making it impossible for memory traces to weaken over time. This enhanced stability doesn’t just preserve memories—it keeps them vivid and easily accessible. As a secondary benefit, the GRIP-enhanced AMPAR network leads to faster neural processing, allowing for quicker access to stored information and improved decision-making under pressure.
Stargazing proteins add an additional layer of reinforcement by ensuring that AMPAR receptors remain in their optimal positions at the synapse. They fine-tune the synaptic landscape, preventing any degradation of signal strength that could otherwise lead to forgetting. This constant support ensures that the electrical and biochemical signals which encode memory remain fully optimized. Beyond memory, stargazing proteins promote neural resilience, protecting synapses from stress and helping the brain recover more quickly from fatigue or cognitive strain.
At the structural level, integrins (α3β1, α5β1, αvβ3) provide the scaffolding necessary for new synaptic connections to be fully anchored. Integrins ensure that the connections between neurons, particularly at newly formed synapses, are not only stable but also resistant to degradation. This ensures that even the most complex memory traces are securely embedded within the brain’s matrix. The secondary benefit of enhanced integrin function is improved neuroplasticity, allowing the brain to reorganize itself more effectively in response to injury or learning new skills, thereby increasing mental agility and the brain’s capacity to rewire itself when needed.
Finally, the subtle electrical current woven into this design provides a continuous low-level stimulation to maintain activity in memory-related circuits. This subtle but constant current ensures that the neuronal networks responsible for memory remain electrically charged and primed for recall. It prevents the natural drift toward dormancy that can occur over time, keeping the brain in a state of heightened readiness. The secondary effect of this current is an overall boost to neural synchrony, improving the brain’s ability to coordinate complex tasks by ensuring that separate regions communicate more fluidly and in sync.
Taken together, this design offers more than just memory enhancement—it’s a complete cognitive upgrade. Each element, from the reactivation of forgotten memories to the solidification of neural networks, works in perfect harmony to create a brain capable of superhuman memory. But beyond memory, these mutations provide a secondary wave of benefits, boosting learning speed, cognitive flexibility, problem-solving, and overall neural resilience. This field isn’t just about remembering more; it’s about transforming the way your brain operates, unlocking the ability to hold vast amounts of data, effortlessly recall information, and adapt to any mental challenge, all with a level of depth and precision never before seen.
All this targeting synapses holding memory traces.